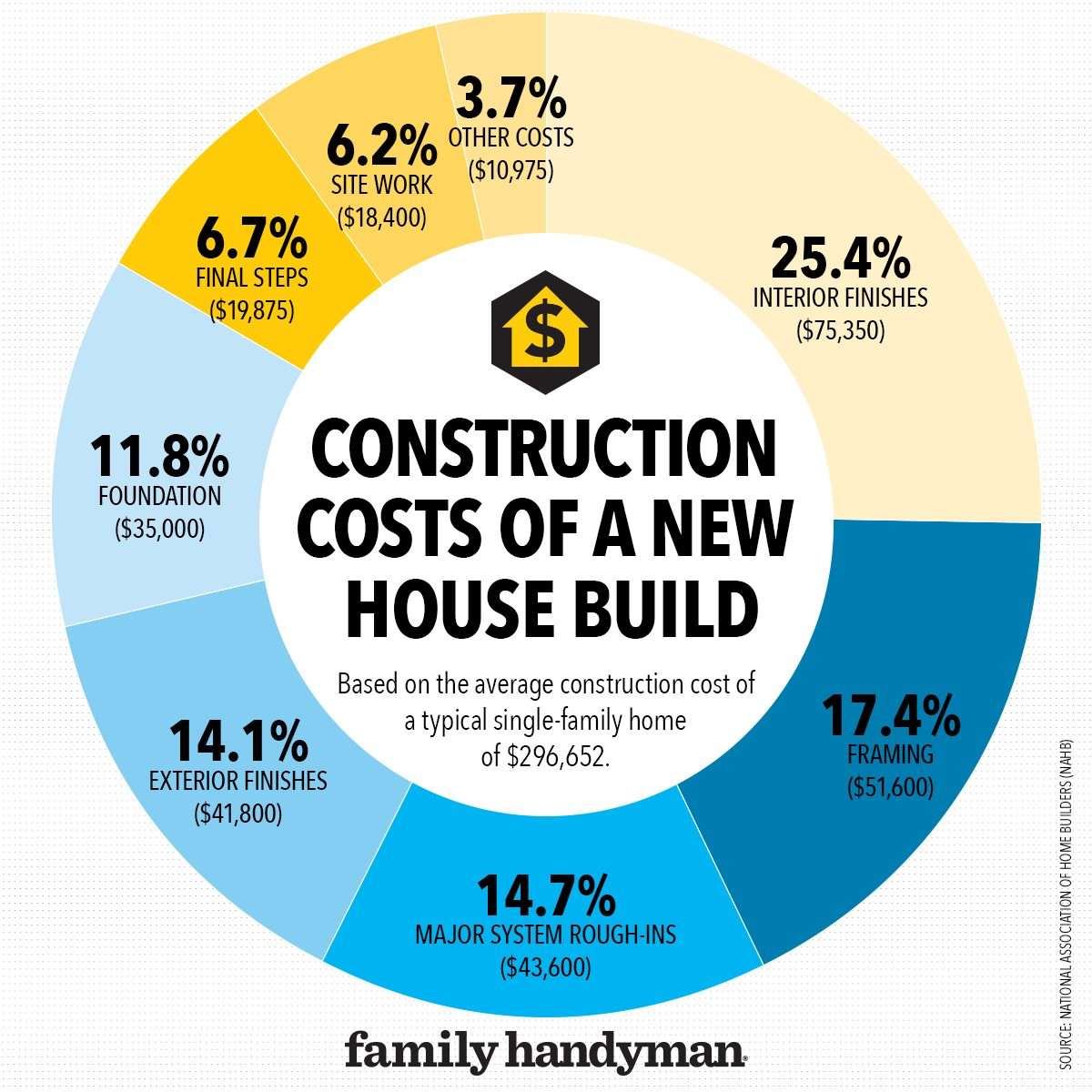
Finding the right building materials is crucial for any construction or renovation project, and when you’re in Thousand Palms, CA, Sepulveda Building Materials stands out as a potential resource. With a reputation built on quality and a selection that caters to various needs, understanding what Sepulveda offers and how it serves the community is essential. This guide aims to provide a comprehensive overview of Sepulveda Building Materials, exploring its products, services, and overall value proposition for residents and contractors in and around Thousand Palms. Making informed decisions about your building supplies is a key step in ensuring the success and longevity of your project.
Exploring the Product Range at Sepulveda
Sepulveda Building Materials likely stocks a wide array of products to meet diverse construction needs. While specifics might vary, common categories often include:
- Lumber: Framing lumber, treated lumber, plywood, and other wood products.
- Concrete & Masonry: Cement, concrete mixes, blocks, bricks, and related supplies.
- Roofing Materials: Shingles, tiles, underlayment, and roofing accessories.
- Drywall & Insulation: Drywall sheets, insulation batts, and related products.
- Hardware: Nails, screws, fasteners, and other essential hardware items.
- Landscaping Supplies: Mulch, gravel, decorative rock, and other landscaping materials (availability may vary).
Beyond the Basics: Specialized Products
Depending on their focus, Sepulveda might also offer specialized products such as:
- Doors & Windows: Pre-hung doors, windows of various styles, and related hardware.
- Plumbing Supplies: Pipes, fittings, valves, and other plumbing components.
- Electrical Supplies: Wiring, conduit, outlets, and other electrical essentials.
Services Offered by Sepulveda Building Materials
Beyond simply selling materials, Sepulveda may offer additional services to enhance the customer experience. These could include:
- Delivery: Bringing materials directly to your job site, a crucial convenience for large projects.
- Cutting Services: Cutting lumber or other materials to your specified dimensions.
- Consultation: Providing expert advice and guidance on material selection and project planning.
- Tool Rentals: Renting out tools for specific jobs.
Why Choose Sepulveda Building Materials in Thousand Palms?
Several factors could influence your decision to choose Sepulveda. Consider these aspects when evaluating your options:
- Price Competitiveness: Compare prices with other local suppliers to ensure you’re getting a fair deal.
- Product Quality: Assess the quality of the materials offered, as this directly impacts the durability of your project.
- Customer Service: Evaluate the helpfulness and expertise of the staff.
- Location & Convenience: Consider the location and ease of access from your project site.
When considering **Sepulveda building materials thousand palms ca**, remember to factor in all these aspects to make the best choice for your specific needs. Ultimately, the goal is to find a reliable supplier that offers quality products, competitive pricing, and excellent customer service.
ASSESSING THE VALUE PROPOSITION OF SEPULVEDA: A DETAILED EXAMINATION
To ascertain the true value proposition presented by Sepulveda Building Materials, a rigorous assessment encompassing several key performance indicators (KPIs) is recommended. This evaluation should extend beyond mere price comparison and delve into the long-term implications of material selection on project longevity and overall cost-effectiveness.
COMPARATIVE ANALYSIS: SEPULVEDA VS. ALTERNATIVE SUPPLIERS
A comprehensive comparative analysis is paramount. This should encompass not only price points but also the quality and certification of materials offered. Scrutinize the origin and manufacturing processes associated with various product lines to ensure adherence to industry standards and regulatory requirements. Furthermore, investigate the availability of warranties and guarantees on specific products, as these can provide crucial safeguards against potential defects or premature failure.
Supplier
Price (Lumber ‒ 2x4x8)
Product Quality (Rating 1-5)
Delivery Options
Customer Service (Rating 1-5)
Warranty Availability
Sepulveda Building Materials
[Insert Price]
[Insert Rating]
[Insert Details]
[Insert Rating]
[Insert Details]
Competitor A
[Insert Price]
[Insert Rating]
[Insert Details]
[Insert Rating]
[Insert Details]
Competitor B
[Insert Price]
[Insert Rating]
[Insert Details]
[Insert Rating]
[Insert Details]
THE SIGNIFICANCE OF CUSTOMER SERVICE AND EXPERTISE
The availability of knowledgeable and responsive customer service personnel constitutes a significant advantage. Personnel equipped to provide informed guidance on material selection, application techniques, and potential challenges can substantially mitigate the risk of errors and optimize project outcomes. Seek out suppliers who offer comprehensive technical support and are demonstrably committed to fostering long-term client relationships.
In conclusion, selecting **Sepulveda building materials thousand palms ca** requires a thorough understanding of their offerings, a careful comparison with competitors, and a keen focus on the long-term implications for your project. By prioritizing quality, value, and expertise, you can ensure a successful and enduring outcome.