The very essence of our planet, from towering mountains to the grains of sand beneath our feet, is profoundly influenced by the fundamental question: what are the building blocks of silicate materials and how do they shape the world around us? These intricate structures, based on silicon and oxygen, form the backbone of a vast array of minerals and rocks. Understanding the intricate arrangement and properties of these building blocks is crucial to comprehending the Earth’s geological processes and the materials we use in everyday life. The **building block of silicate materials** dictates not only their physical properties but also their chemical behavior, impacting everything from the formation of fertile soil to the development of advanced ceramics. So, let’s delve into the fascinating world of silicate chemistry and explore the impact of **building block of silicate materials**.
The Tetrahedral Foundation: SiO4
At the heart of every silicate mineral lies the silicon-oxygen tetrahedron (SiO4). This fundamental unit consists of a silicon atom bonded to four oxygen atoms, forming a pyramid-like structure. The arrangement of these tetrahedra, and how they link together, dictates the overall structure and properties of the resulting silicate material.
Isolated Tetrahedra (Nesosilicates)
In some silicate minerals, the tetrahedra remain isolated, not sharing any oxygen atoms with neighboring tetrahedra. These are known as nesosilicates, or island silicates. Examples include:
- Olivine: A common mineral found in Earth’s mantle.
- Garnet: Used as a gemstone and an abrasive.
Linking the Tetrahedra: A Variety of Structures
The magic of silicate minerals lies in the diverse ways the SiO4 tetrahedra can link together. Sharing oxygen atoms creates chains, sheets, and three-dimensional frameworks, each with distinct properties.
Chain Silicates (Inosilicates)
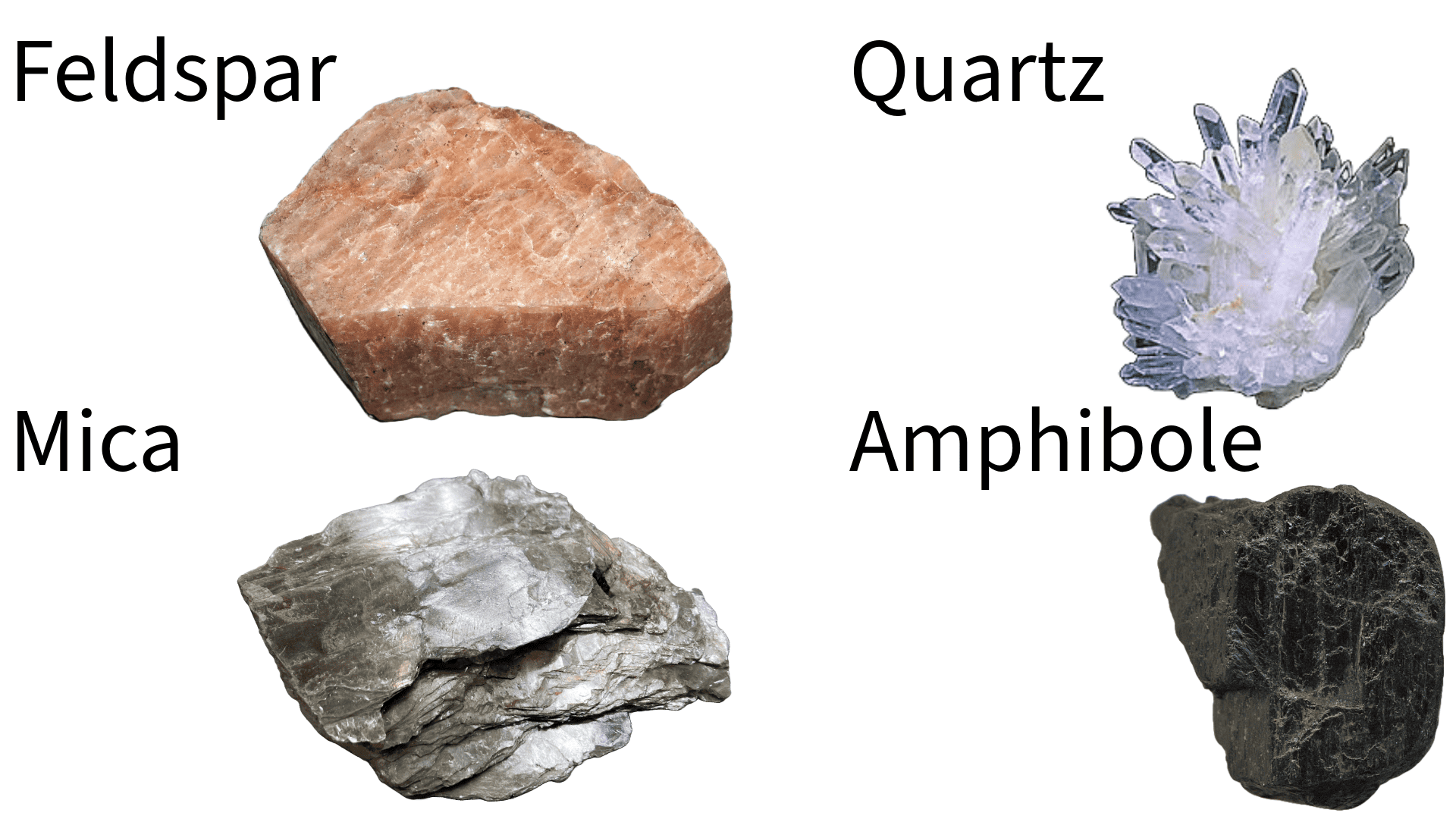
When tetrahedra share two oxygen atoms, they form chains. These chains can be single (pyroxenes) or double (amphiboles). The **building block of silicate materials** arranged in chains results in minerals that tend to be elongated and cleave along the chain direction.
Sheet Silicates (Phyllosilicates)
Sharing three oxygen atoms creates sheets. These sheet silicates, like mica and clay minerals, have a layered structure that allows them to easily cleave into thin sheets. The layered structure is key to their use in many products.
Framework Silicates (Tectosilicates)
The most complex silicate structures are framework silicates, where each tetrahedron shares all four of its oxygen atoms with neighboring tetrahedra. Quartz and feldspar are prime examples of framework silicates. These minerals tend to be hard and resistant to weathering.
Impact on Material Properties
The structure of silicate materials directly influences their physical properties, such as hardness, cleavage, and melting point. For instance:
- Minerals with isolated tetrahedra tend to be harder and have no distinct cleavage.
- Chain silicates tend to be prismatic and have two directions of cleavage.
- Sheet silicates have perfect cleavage in one direction, allowing them to be easily split into thin sheets.
- Framework silicates are hard and have poor or no cleavage.
Understanding the way that the building blocks assemble allows us to understand the diversity of materials we see on earth and use in every day products.
THE BUILDING BLOCK OF SILICATE MATERIALS: SHAPING OUR WORLD
The very essence of our planet, from towering mountains to the grains of sand beneath our feet, is profoundly influenced by the fundamental question: what are the building blocks of silicate materials and how do they shape the world around us? These intricate structures, based on silicon and oxygen, form the backbone of a vast array of minerals and rocks. Understanding the intricate arrangement and properties of these building blocks is crucial to comprehending the Earth’s geological processes and the materials we use in everyday life. The **building block of silicate materials** dictates not only their physical properties but also their chemical behavior, impacting everything from the formation of fertile soil to the development of advanced ceramics. So, let’s delve into the fascinating world of silicate chemistry and explore the impact of **building block of silicate materials**.
THE TETRAHEDRAL FOUNDATION: SIO4
At the heart of every silicate mineral lies the silicon-oxygen tetrahedron (SiO4). This fundamental unit consists of a silicon atom bonded to four oxygen atoms, forming a pyramid-like structure. The arrangement of these tetrahedra, and how they link together, dictates the overall structure and properties of the resulting silicate material.
ISOLATED TETRAHEDRA (NESOSILICATES)
In some silicate minerals, the tetrahedra remain isolated, not sharing any oxygen atoms with neighboring tetrahedra. These are known as nesosilicates, or island silicates. Examples include:
– Olivine: A common mineral found in Earth’s mantle.
– Garnet: Used as a gemstone and an abrasive.
LINKING THE TETRAHEDRA: A VARIETY OF STRUCTURES
The magic of silicate minerals lies in the diverse ways the SiO4 tetrahedra can link together. Sharing oxygen atoms creates chains, sheets, and three-dimensional frameworks, each with distinct properties.
CHAIN SILICATES (INOSILICATES)
When tetrahedra share two oxygen atoms, they form chains. These chains can be single (pyroxenes) or double (amphiboles). The **building block of silicate materials** arranged in chains results in minerals that tend to be elongated and cleave along the chain direction.
SHEET SILICATES (PHYLLOSILICATES)
Sharing three oxygen atoms creates sheets. These sheet silicates, like mica and clay minerals, have a layered structure that allows them to easily cleave into thin sheets. The layered structure is key to their use in many products.
FRAMEWORK SILICATES (TECTOSILICATES)
The most complex silicate structures are framework silicates, where each tetrahedron shares all four of its oxygen atoms with neighboring tetrahedra. Quartz and feldspar are prime examples of framework silicates. These minerals tend to be hard and resistant to weathering.
IMPACT ON MATERIAL PROPERTIES
The structure of silicate materials directly influences their physical properties, such as hardness, cleavage, and melting point. For instance:
– Minerals with isolated tetrahedra tend to be harder and have no distinct cleavage.
– Chain silicates tend to be prismatic and have two directions of cleavage.
– Sheet silicates have perfect cleavage in one direction, allowing them to be easily split into thin sheets.
– Framework silicates are hard and have poor or no cleavage.
Understanding the way that the building blocks assemble allows us to understand the diversity of materials we see on earth and use in every day products.
In conclusion, the impact of the silicate tetrahedron as **the building block of silicate materials** is undeniable. From the Earth’s crust to the technologies we rely on, the structure and arrangement of these fundamental units play a critical role. Further research into the intricacies of silicate chemistry promises to unlock new materials and technologies for the future.
PRACTICAL APPLICATIONS AND CONSIDERATIONS
Now that we’ve explored the fascinating architecture of silicate materials, let’s turn our attention to their practical implications. Understanding these structures allows us to better utilize them in a variety of industries. From construction to electronics, silicates are indispensable. Here’s some advice on how to think about silicates in your own projects and applications:
CONSTRUCTION AND INFRASTRUCTURE
Concrete, a cornerstone of modern construction, relies heavily on silicate minerals. Portland cement, the binding agent in concrete, is produced by heating a mixture of limestone and clay, both rich in silicates. Consider the following:
– Durability: The type of silicate minerals used in cement production greatly affects the concrete’s durability. Ensure the appropriate blend is chosen based on environmental factors and expected loads.
– Additives: Incorporating supplementary cementitious materials (SCMs) like fly ash or silica fume can enhance the concrete’s strength, workability, and resistance to chemical attack.
– Sustainability: Explore the use of alternative cements that reduce the environmental impact associated with traditional Portland cement production.
CERAMICS AND GLASS
Ceramics and glass are primarily composed of silicate minerals. Their properties can be tailored by controlling the composition and processing techniques. Keep these points in mind:
– Thermal Properties: The high thermal stability of silicate ceramics makes them ideal for applications involving high temperatures, such as furnace linings and heat shields.
– Optical Properties: By carefully controlling the composition of glass, we can achieve a wide range of optical properties, from transparent windows to colored lenses.
– Recycling: Glass is highly recyclable, reducing the demand for virgin materials and conserving resources.
AGRICULTURE AND ENVIRONMENTAL SCIENCE
Silicate minerals play a crucial role in soil formation, fertility, and water filtration. Here’s some guidance:
– Soil Fertility: Weathering of silicate minerals releases essential nutrients like potassium and calcium, enriching the soil and supporting plant growth.
– Water Filtration: Certain silicate minerals, such as zeolites, have excellent adsorption properties, making them effective for removing pollutants from water.
– Soil Amendment: Applying silicate amendments can improve soil structure, water retention, and nutrient availability, leading to increased crop yields.
EMERGING TECHNOLOGIES
Research into novel silicate materials is constantly pushing the boundaries of technology. Consider these emerging areas:
– Geopolymers: These materials offer a low-carbon alternative to cement and can be used in a wide range of applications, from construction to waste stabilization.
– Nanomaterials: Silicate nanoparticles are being explored for drug delivery, catalysis, and energy storage.
– 3D Printing: Silicate-based materials are increasingly being used in 3D printing for creating complex and customized structures.
By understanding the fundamental principles governing the structure and properties of silicate materials, we can unlock their full potential and create innovative solutions to address global challenges. Stay informed, experiment with new materials, and continue to explore the fascinating world of silicate chemistry. Always prioritize safety and consult with experts when working with new or unfamiliar materials. The future of materials science is intertwined with our understanding of these fundamental building blocks.